Name that atom worksheet answers – Welcome to the realm of atomic identification, where the “Name That Atom” worksheet reigns supreme. This comprehensive guide will equip you with the knowledge and skills to navigate the intricacies of this essential tool, empowering you to confidently decipher the secrets of the atomic world.
Embark on a journey through the periodic table, unraveling the mysteries of protons, neutrons, and electrons. Discover the significance of atomic number and mass number, and witness the fascinating process of ion formation. Along the way, practical applications and real-world examples will illuminate the profound impact of atom identification in diverse fields, from chemistry to medicine.
Name That Atom Worksheet Answers
The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. It is generally accepted that the modern periodic table was first published by Dmitri Mendeleev in 1869, although several other scientists had developed similar tables prior to this.
The periodic table is a powerful tool for identifying atoms because it provides a wealth of information about each element. The atomic number of an element is the number of protons in its nucleus, and it determines the element’s position in the periodic table.
The mass number of an element is the sum of the number of protons and neutrons in its nucleus, and it is also listed in the periodic table.
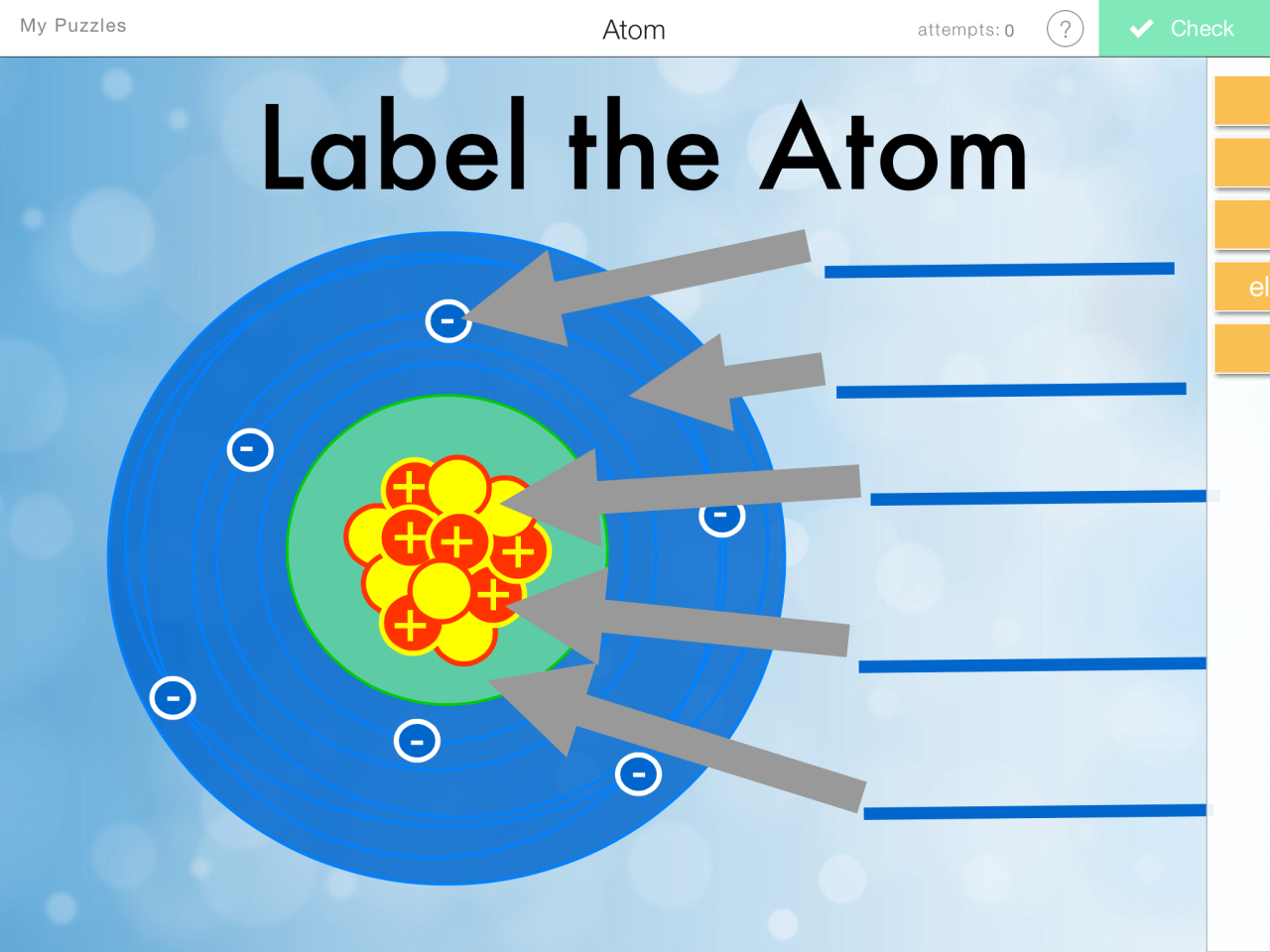
Atoms are the basic building blocks of matter. They are composed of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge.
The number of protons in an atom’s nucleus determines its atomic number, and the number of electrons in an atom determines its chemical properties.
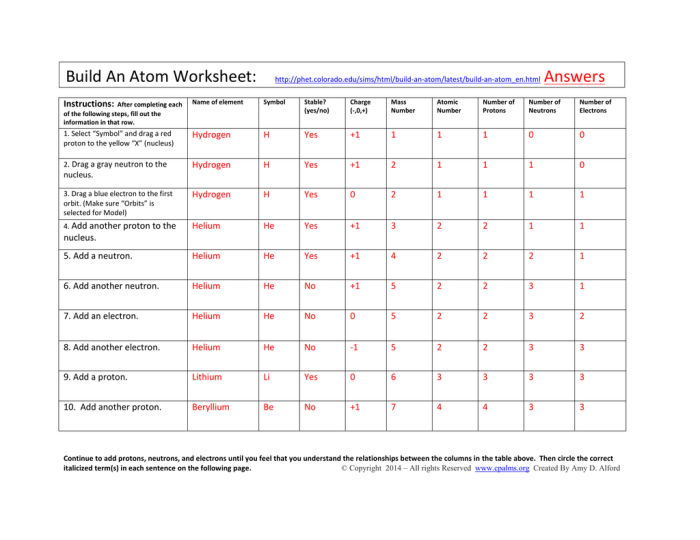
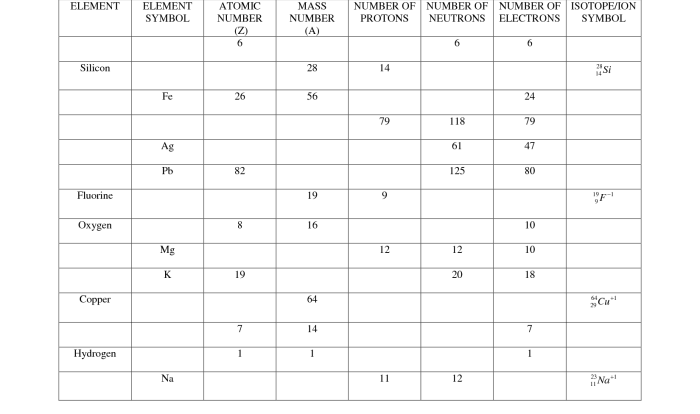
A “name that atom” worksheet is a type of practice worksheet that helps students learn to identify atoms. These worksheets typically provide students with a list of atomic numbers, mass numbers, and electron configurations, and ask them to identify the corresponding element.
Worksheets of this nature are a valuable tool for reinforcing atomic structure concepts.
Worksheet Structure and Content, Name that atom worksheet answers
A typical “name that atom” worksheet will include the following sections:
- A table of atomic numbers, mass numbers, and electron configurations
- A list of element symbols
- Instructions for completing the worksheet
The table of atomic numbers, mass numbers, and electron configurations provides the basic information needed to identify each atom. The list of element symbols provides the possible answers to the worksheet questions. The instructions for completing the worksheet typically explain how to use the periodic table to identify each atom.
Methods for Identifying Atoms
To identify an atom using a “name that atom” worksheet, follow these steps:
- Find the atomic number of the atom in the table.
- Use the periodic table to find the element with the corresponding atomic number.
- Write the element symbol of the atom in the space provided.
For example, if the atomic number of an atom is 6, then the element is carbon. The element symbol for carbon is C.
Electron Configuration and Ion Formation
Electron configuration is the distribution of electrons in the orbitals of an atom. It is important for atom identification because it determines the chemical properties of an atom.
When an atom loses or gains electrons, it becomes an ion. Ions have a net positive or negative charge. The charge of an ion is determined by the number of electrons that the atom has lost or gained.
Electron configuration can be used to predict the charge of an ion. For example, if an atom has lost one electron, then it will have a net positive charge of 1. If an atom has gained one electron, then it will have a net negative charge of 1.
Applications and Examples
Atomic identification is used in a variety of fields, including chemistry, medicine, and materials science.
In chemistry, atomic identification is used to determine the composition of compounds and to predict their chemical properties. In medicine, atomic identification is used to diagnose and treat diseases. In materials science, atomic identification is used to develop new materials with improved properties.
One example of the practical application of atomic identification is in the field of forensics. Forensic scientists use atomic identification to identify the elements present in a sample of evidence. This information can be used to link a suspect to a crime scene or to identify the victim of a crime.
Helpful Answers: Name That Atom Worksheet Answers
What is the purpose of a “Name That Atom” worksheet?
These worksheets provide practice in identifying atoms based on their atomic number, mass number, and electron configuration, reinforcing key concepts of atomic structure.
How do I use the periodic table to identify an atom?
The periodic table organizes elements based on their atomic number, allowing you to determine the element symbol and atomic mass of an atom based on its position in the table.
What is the significance of electron configuration in atom identification?
Electron configuration describes the arrangement of electrons in an atom’s orbitals, providing insights into its chemical properties and potential for ion formation.